There is a lot of uncertainty in the world right now, but one thing for certain is that rare disease and orphan indication clinical research must go on. Medpace can support your clinical studies with a full suite of services, expertise, and technology and has kept moving with rare disease research during COVID-19. Our MDs, operations, labs, and imaging experts are ready to tackle tough development projects no matter what.
Our Focused Rare Disease Team Delivers Results
- Committed, cross-functional team of Rare Disease experts comprised of doctors, nurse practitioners, project managers, seasoned clinical operations, and regulatory experts to orchestrate these complex studies including advanced therapies
- Board-certified Pediatricians with backgrounds treating pediatric patients with Rare Disease / Orphan Drug conditions
- Site relationships with access to patient registries to drive patient enrollment
- Experience in the planning and management of advanced therapy clinical trials with proactive solutions to the operational complexities inherent in these therapies
- Supports the collection and analysis of patient-reported data through TrialPACE™ ePRO/eCOA/eDiary system
- Successful and expedited patient recruitment through IntelliPACE®️, Medpace’s in-house program combining data-driven strategy with customized execution for rare disease studies
- Broad understanding of patient advocacy issues and organizations for support
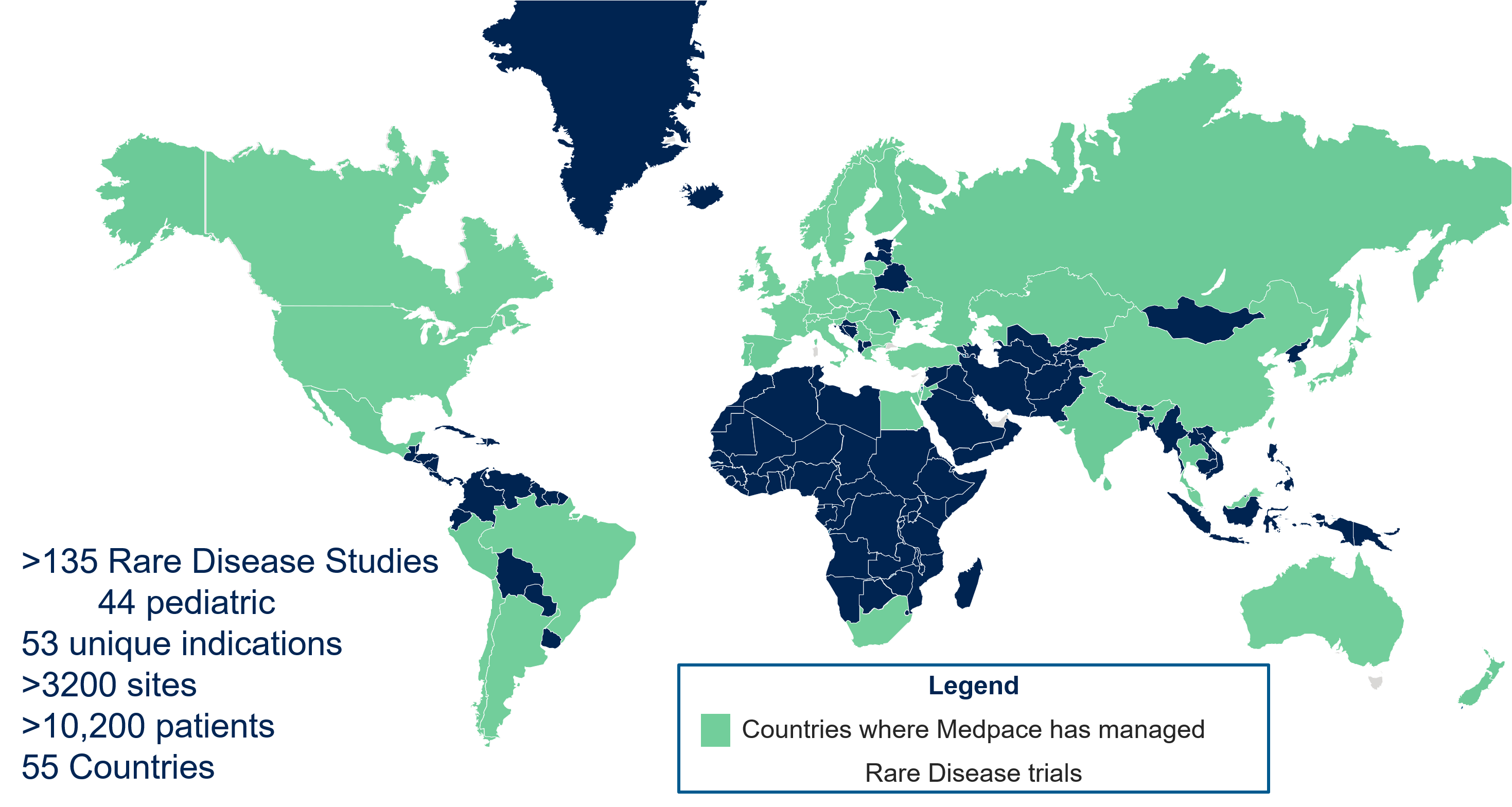
- Global experience managing rare disease trials
Thought Leadership from our Experts
- Whitepaper: Lessons Learned In Emerging Cell And Gene Therapies: A Holistic Approach To Clinical Development Read Now
- Article: Regulatory Strategies And Considerations For Orphan And Pediatric Drug Designations Read Now
- Article: Patient And Caregiver-Centered Approaches In Rare Disease Clinical Trials Read Now
- Webinar: Part 1: Rare Disease Clinical Research – Spotlight on the Patient and Caregiver Watch Now
- Webinar: Part 2: Rare Disease Clinical Research – A Deep Dive into the Regulatory Strategies & Considerations Watch Now
- Webinar: Part 3: Rare Disease Clinical Research – Strategies for Ensuring Endpoint Integrity Watch Now
Learn more about Medpace’s Rare Disease capabilities.