
Operational Experts Share Highlights from OCT East Asia 2023
In December 2023, Medpace medical and operational experts attended the Outsourcing Clinical Trials (OCT) East Asia conference in Seoul, South Korea. Learn more from Shinwook Nam, Senior Associate Director, Clinical Trial Management, as she recaps highlights from the conference.
Asia Pacific Insights for Global Drug Development
Navigating Landscape, Clinical Considerations, and the Role of CROs
In the era of innovation for emerging biotech drug development, Asia Pacific is taking control of drug development pipelines and establishing itself as a pivotal force in global biotech innovation. Despite the biotech slowdown, Asia Pacific exhibits significant potential growth over the next 3-5 years, surpassing global growth rates. Amidst this monumental progress, Asia Pacific faces evolving challenges in clinical development. OCT East Asia incorporated a variety of topics addressing the various challenges, including limited therapeutic expertise, regulatory complexities, laboratory management, and operational hurdles. Throughout these discussions, an underlying theme emerged – the imperative for collaboration in Asia Pacific clinical development.
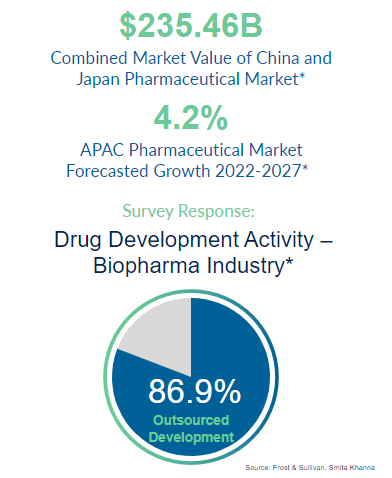
2024 Strategy Considerations
Therapeutic Development
Strategic Planning Throughout Therapeutic Development
Novel therapies like oncology, cell and gene therapy, and CNS in Asia Pacific demand strategic planning for successful development. When designing your clinical trial, embed flexibility during your strategic planning period. Design manufacturing and non-clinical drug programs to ensure you can get into the clinical phase, establish early development targets, follow phase-appropriate development, and allocate resources accordingly.
In the evolving landscape of therapeutic development, benefit from strategic CRO partnerships that specialize in complex modalities and evolving therapeutic areas, in addition to global experience and capabilities.
Regulatory Affairs
Early Scientific Advice for RA (EU, US, and Asia Pacific)
Regulatory processes for global clinical trials can be complex. In the Asia Pacific region, the clinical development industry poses challenges, equating to a complicated regulatory landscape, impacting biotech innovation. In order to overcome regulatory hurdles, biopharma companies must obtain early alignment with authorities to prevent development delays. When working with such state-of-the-art technologies, there are often no defined regulatory processes in place. In this case, it is essential to work in close collaboration with regulatory agencies. Additionally, biopharma companies should try to understand the competitive landscape – namely, whether it is the first in that space or whether there are other competitors in the space, but none have approved or marketed the technology yet.
For FDA or EU approval, a strategic CRO partner can provide needed experience and expertise, ensuring efficient navigation and minimizing time with with the regulatory authorities due to improper planning.
Laboratory Management
The Role of Biomarkers in Lab Management
Strategic planning involves a number of different compound development stages. These include the establishment of medical or laboratory strategy, in addition to therapeutic development strategy and regulatory planning. Identifying the correct biomarkers is critical to a successful laboratory strategy. A biomarker can support efficacy studies, eliminate unnecessary testing, help identify and understand patient populations, support the choice of different cell lines, and make drugs safer.
A laboratory partner can support biotech in determining the most suitable biomarkers, identifying the best regulatory approach, and developing solid plans for study execution. Medpace’s strategically located network of global laboratories can support global development, including US, Europe, and Asia Pacific operations.
Operational Considerations
Operational Excellence in Global Clinical Development
Clinical trial management is critical to meeting milestones and ensuring quality throughout your clinical development. When considering full-service versus functional strategies, it is critical to evaluate the operational methodology’s impact on your clinical development. As the nature of clinical trials continue to evolve, and as regulatory guidelines become more complex, choosing an operational approach can have a major impact on development timelines, cost, and quality of results.
At Medpace, our full-service operating model has been refined over three decades of experience successfully executing the most scientifically and operationally challenging clinical trials, without the disruption of mergers and acquisitions that are so common in the industry. Benefit from our full breadth of services, cross-functional expertise, global experience, essential partnerships, in a centralized fashion – through one point of contact.
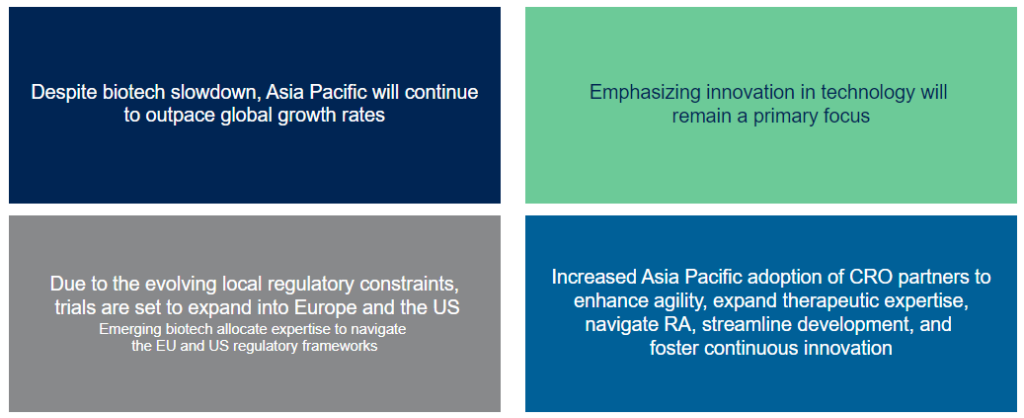
2024 Strategy Considerations
Interested in learning more? Watch our on-demand webinar, “2024 Asia Pacific Insights for Drug Development – Exploring Landscape, Clinical Considerations, and the Role of a CRO.”
Asia Pacific Drug Development Services
As a global CRO with an operational footprint across 40 countries, Medpace has broad experience designing and conducting Phase I-IV clinical trials around the world. Our medical, regulatory, and operational experts have the resources to advance your medical therapeutic in any region. Since establishing our Asia Pacific presence in 2004, our medical and operational specialists have country-specific expertise, which allows them to integrate local language, culture, and requirements into study conduct, to deliver faster enrollment, and obtain access to country-specific patient populations. Our regulatory experts can plan and coordinate each aspect of regulatory strategy and engagement – locally and globally.