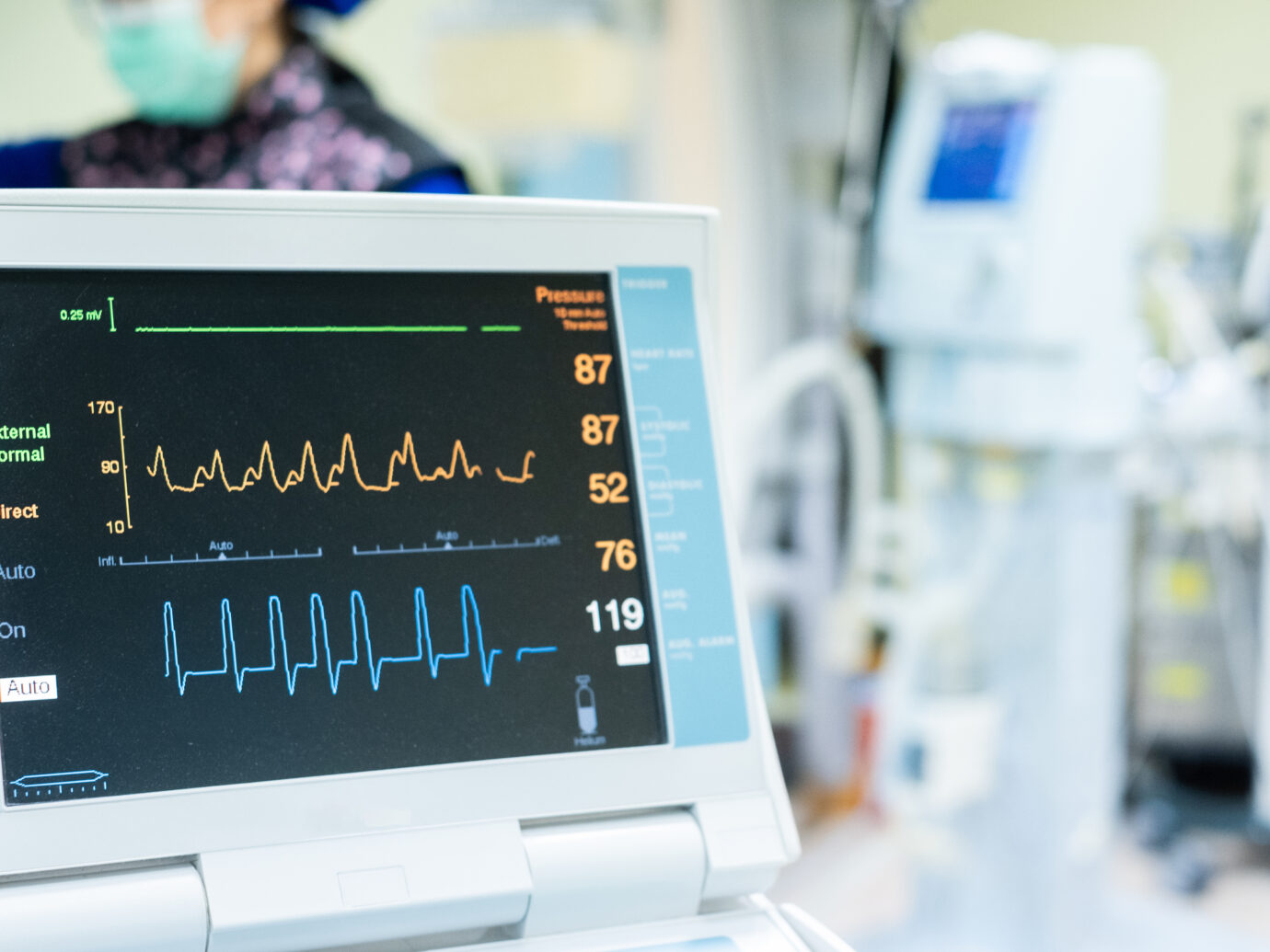
Integrated Medpace Cardiovascular & ECG Core Lab
Medpace Core Lab supports global Phase I-IV clinical trials through the integration of standardized cardiac safety equipment into the complex structure of the overall clinical trial.
The Medpace Cardiovascular Core Lab provides the full spectrum of cardiovascular monitoring capabilities for clinical trials optimized for over 30 years, routinely collaborating with the Medpace CRO on managed studies requiring central core lab services.
Key Cardiovascular Capabilities
Cardiovascular core lab services can be used as an integral part of the Medpace full-service CRO product offering or as a stand-alone service. MCL resources and ECG support services for Phase I-lll global studies include:
- ECG, ABPM, and Holter equipment and logistics as well as data collection and interpretation, including TQT reporting
- In-house medical expertise; physicians and other cardiology specialists on staff with excellent peer-to-peer relationships with KOLs
- Global experience with trial design, data interpretation and analysis, study management, and regulatory strategy
- Acquisition and analysis services utilizing industry standard devices, systems, and expert central reviewers: results interpreted in the context of all other trial data
- On-campus, Cincinnati-based 60,000 sq. ft. Phase I Unit
Innovative Wearable Biosensor Technology Capabilities
Medpace Core Lab has extensive experience supporting Sponsors through the integration of wearable devices into the complex structure of the overall trial. Medpace seamlessly collects, harmonizes, and integrates data from wearables and remote devices, such as ECGs, into your clinical study as part of our full-service offering. Wearable biosensor technologies are selected and vetted by Medpace’s therapeutically-aligned teams to meet tailored study design requirements, streamline vendor management, and accelerate study start-up.
Medpace Cardiovascular Core Lab provides centralized acquisition and analysis of:
Streamlined Process
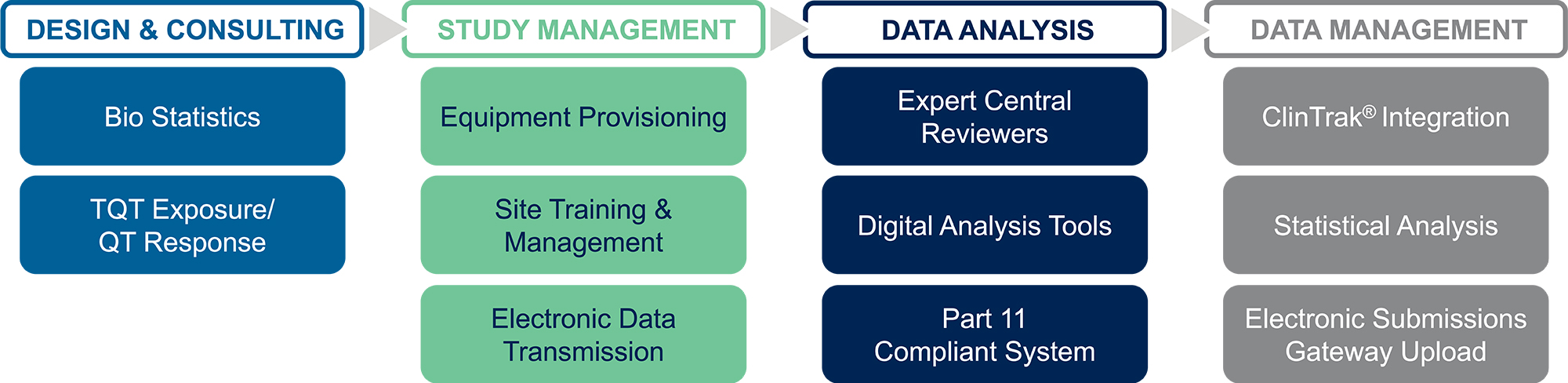
Design and Consulting
- Protocol design and consulting with Medpace medical, statistical, and regulatory specialists ensure all aspects of cardiac safety and efficacy
- The same team can provide protocol review services and suggest efficiencies or improvements to an existing design, resulting in reduced budgets and optimized data collection/power for regulatory submission
- Medpace provides consulting from our experts with extensive experience in the design of TQT studies including development of TQT Waiver applications and Thorough QT analysis, interpretations and reports to regulatory authorities
- Assistance in determining candidacy for alternative Exposure/QT response study to obviate the need for a TQT analysis; saving time and money in establishing the cardiac safety profile of your compound
Study Management
- Medpace Core Labs manages global clinical trials in over 60 countries to ensure your study will receive the attention needed, should higher than normal levels of support be required
- Medpace global equipment logistics experience ensures your devices will arrive at investigator sites when and where needed
- Localized training support keeps sites engaged and educated in proper acquisition and transmission of critical study data
- Localized Medpace site support can be employed to resolve any issues that may arise during the course of a study
- 24x7x365 technical support to ensure timely resolution of support needs
- Industry standard ECG and blood pressure devices provide validated performance for high quality data
- Electronic transmission of data allows expedited receipt of digital data for timely analysis
Data Analysis
- Expert central reviewers ensure high quality, expert analysis to meet the skilled readers needs of your study
- Industry standard electronic analysis tools allow precision that paper-based studies can’t provide
- Medpace validation of systems ensures proper qualification of tools and methodology
- 21 CFR part 11 compliant systems ensure every action taken within the analysis of data is electronically documented and can be reviewed upon request
Data Management and Submission
- ClinTrak® database integration provides review of results in the same system as all other study parameters contracted with Medpace for a comprehensive view of study progression and outcomes
- Reporting of statistical results and medical conclusions with medical writing services needed for regulatory submission, and, if required, work with your team to present the data to regulatory bodies
- Medpace Cardiovascular Core Lab can upload TQT and Exposure/QT response data into the FDA’s Electronic Submissions Gateway, as well as providing access to the data and review tools the FDA reviewers will use, prior to actual data submission
Phase I/TQT/Exposure/QT Response
ICH E14 acceptance of Exposure-QT Response analysis provides a cost-effective alternative to formal TQT studies. Medpace Core Lab understands the current regulatory environment and can ensure a well-designed study with high-quality cardiac safety assessments in a regulatory compliant environment.
Medpace Phase I Unit
Located on the same campus as Medpace Core Lab, the Medpace Phase I Unit provides an optimal environment for cardiac safety studies. The Phase l Unit is sensitive to requirements surrounding ECG data and ensures that data are available for analysis on-demand.
The Phase I Unit utilizes 12-lead telemetry system to provide the highest quality ECG data available for complex, ECG intense studies. This provides the core lab with high resolution, continuous 12-lead ECG data to allow for the detection of a 1ms change, with a 10ms shift from baseline as the threshold for regulatory concern.
Phase I Highlights:
- Board-certified cardiologists who follow strict quality assurance and confidentiality standards to provide FDA-, EMEA-, and ICH-compliant ECG safety analysis to read every digital ECG
- Sponsor access to ECG data via web-based, Medpace ClinTrak DM
- State-of-the-art validated technologies that meet international regulatory requirements