
Improving Healthcare for Women Across the Globe
Clinical research for women’s health has garnered attention from the pharmaceutical research and drug development space, but is much more than a buzzword. Despite impacting nearly half of the population, women’s health clinical development has increased in complexity, facing hurdles such as steep competition for site resources, patient recruitment challenges, and insufficient cross-therapeutic expertise.
At Medpace, we have a therapeutically aligned leadership team focused specifically on improving women’s health clinical research. We understand the challenges specific to women’s health clinical trial development, informed by our experience across a broad range of therapeutic areas and indications including oncology, infectious diseases, and metabolic disorders. Ultimately, we at Medpace strive to fill the many unmet needs in women’s health and improve healthcare outcomes and quality of life for women across the globe.
Diverse Therapeutic Expertise
The intersection of Women’s Health and related diverse disciplines is handled through the collaboration of our medical leadership and functional areas. Medpace accelerates Women’s Health development by fully embedding medical leadership and a wide array of relevant therapeutic expertise, integrated across all functional areas, into the clinical trial process. This cross-therapeutic and cross-functional area collaboration allows for optimized trial communication and execution, and ultimately, expedited timelines.
Medpace Women’s Health experience over the past 5 years:
Making the Complex Seamless®
in Global Women’s Health
Clinical Trials
The Medpace women’s health team has a deep understanding of the complexity of managing clinical trials in a broad range of conditions, having contributed to over 75 women’s health clinical studies in recent years.
For over 30 years, Sponsors across emerging biotechnology and global pharmaceutical companies have trusted Medpace to lead their women’s health clinical development.

Capabilities Overview
Gain a competitive edge for Women’s Health Clinical development
Women’s Health Leadership Team
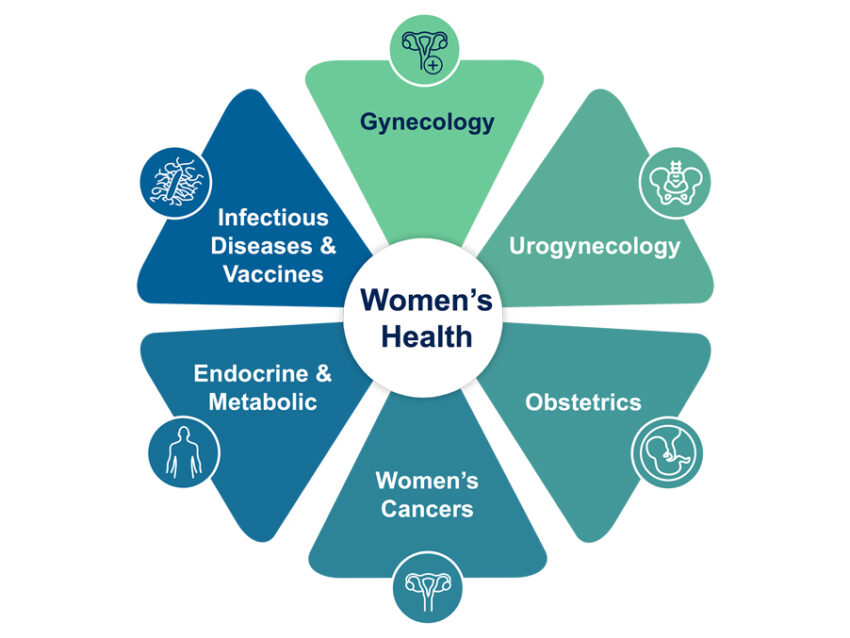
At Medpace, we define Women’s Health as a multifaceted field encompassing various therapeutic areas critical to addressing the unique healthcare needs of women and individuals with female biologies. Our female-led Women’s Health Leadership team consists of therapeutically-aligned medical and operational experts who possess deep clinical knowledge of Women’s Health indications, study design and execution.
Cross-Functional Support
By integrating medical leadership across all functional areas, we optimize trial communication and execution, leading to expedited timelines and improved outcomes.
Defining Women’s Health
Women’s Health CRO Capabilities
When you partner with Women’s Health CRO Medpace, medical leadership is fully embedded within the clinical trial process from inception to completion to help accelerate development. Your trial will benefit from our in-depth knowledge of disease processes and pathophysiology unique to women, relevant outcome measures, and standards of care.
Therapeutically-Aligned Leadership Team
Medpace medical leadership and expertise in Women’s Health spans multiple therapeutic areas and indications, including Gynecology, Urogynecology, Obstetrics, Oncology, Endocrinology and Metabolic Disease, and Infectious Disease and Vaccines (ID&V).
Obstetrics, Gynecology & Urogynecology Expertise
At Medpace, our Women’s Health experts possess extensive expertise in gynecologic and urogynecologic conditions. Our distinguished Women’s Health medical team provide leadership across a wide spectrum of these disciplines, ensuring comprehensive care and innovative approaches. With a combination of deep clinical knowledge and robust research experience, we excel in designing and executing gynecologic and urogynecologic clinical trials.
Women’s Infectious Diseases & Vaccines
Our Medpace ID&V team has extensive experience in designing and conducting infectious disease clinical trials. Our experts have broad clinical experience in managing the full spectrum of infectious diseases which can disproportionately affect women and have additional downstream complications. Our team fully understands the unique challenges to running infectious disease trials in women. Our combined expertise in Women’s Health, Infectious Disease and Vaccines translates into optimal study design and execution with the goal of continued advancement for the prevention and treatment of infectious diseases in women.






