
Accelerate Study Activation
Working closely with our therapeutic and regulatory experts, our dedicated team members engage quickly and provide strategic thinking to ensure swift start-up times. Our strategic regional presence throughout North America, EMEA, Asia-Pac and Latin America ensures you are benefiting from global expertise along with local knowledge—from working with regulatory authorities and ethics committees to drafting informed consent forms and negotiating site contracts and budgets. Our approach to study start-up is:
- Proactive: The team at Medpace is committed to ensuring your trial is initiated on-time and on-budget. Country-specific and regional regulatory expertise enables us to develop proactive solutions to regulatory issues and challenges within achievable timelines. Our start-up specialists coordinate and oversee study submissions to Regulatory Authorities and Ethics Committees, facilitate essential document collection, and review and finalize contracts.
- Customized: Every study has its own set of unique challenges. When you partner with Medpace, you get a customized approach designed specifically to meet your timelines. Our start-up teams craft individual strategic plans for country-specific submissions, informed consent form (ICF) negotiations, contract and budget negotiations, and translation procedures to accelerate site initiations.
- Experienced: Our start-up teams have initiated clinical trials in over 60 countries and bring a diverse combination of clinical operations and industry experience to your clinical trial.
Our Process: Customized Start-Up Strategy & Execution
We offer a breadth of global capabilities and experience to execute your customized start-up strategy. Our start-up specialists are dedicated to the trial before, during, and after site initiations, ensuring any amendments to your protocol are managed seamlessly without impacting study participation.
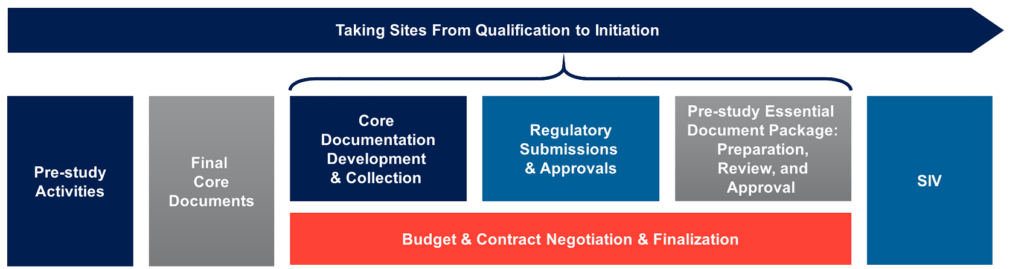

Managing Site Activations
Your Regulatory Submissions Manager (RSM) will be your single point of contact for study start-up and regulatory maintenance throughout the life of your trial. Responsible for the development and execution of the start-up strategy, your RSM will collaborate with the full team of specialists to expedite start-up activities in each country and project start-up timelines for each site. Unlike many other CROs, these specialists remain assigned to the project for its duration, which provides continuity and familiarity should there be any changes such as amendments, or new sites/countries added.

