Medical, Scientific and Technical Expertise in
Neuroimaging for Clinical Trials
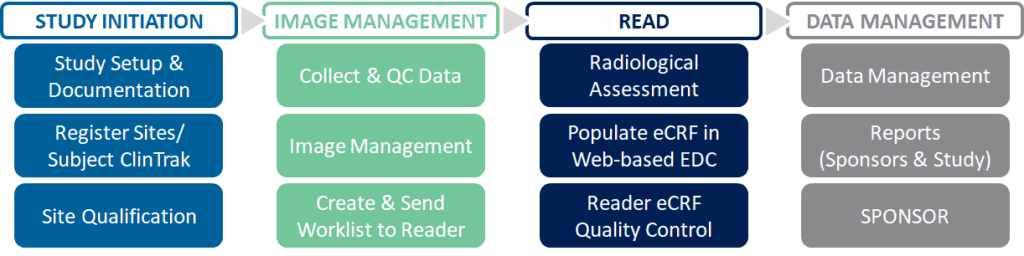
Medpace neuroscience imaging is supported by an integrated team comprised of scientists, clinicians, technologists, project managers, and coordinators. Our team of experienced professionals brings training and board certification in MRI, CT, SPECT/PET, Ultrasound, and experience to clinical trials using neuroimaging biomarkers. Our imaging project managers have decades of experience in managing teams and processes for all aspects of neurodevelopmental, neurodegenerative, and neuromuscular disorder trials; including site qualification, image acquisition, reading, analysis, quality checks, data management, and documentation.
Team members include:
- Clinicians: Board-certified radiologists and neurologists with extensive clinical trial experience
- Scientists: Physicists and engineers with PhD and MS level training covering all neuroimaging modalities and analysis methods
- Imaging Technologists: Clinically trained and certified with clinical trial experience across modalities
- Project Managers: Specialized training and skills for managing neurodevelopmental, neurodegenerative, and neuromuscular disorder trials
- Project Coordinators: Experienced in Medpace processes for clinical trial operations, documentation, and quality assurance
Streamlined Process
- Centralized process for the management and execution of neuroimaging trials with global and local sites
- 24/7 global site management
- Imaging Review Charter (IRC) and other documentation
- Rigorous quality control and validation process
- Compliant with Metrics Champion Consortium (MCC)
Expert Quality
- Standard operating procedure (SOP) driven processes
- CFR Part 11 compliant & fully-validated, vendor-neutral imaging systems for clinical trials
- Dedicated imaging quality assurance (QA) officers and processes
- Validated customized image analysis tools
- Qualified sites, qualified readers, professional management
Neurology Imaging Experience
Experience
- Neurodevelopmental disorders
- Pediatric rare diseases
- Metabolic disorders
- Ataxias
- Neurodegenerative Disorders
- Multiple Sclerosis
- Bipolar Disorder, Schizophrenia, & Mental Health
- Neuro-oncology
- Parkinson’s Disease
- Huntington’s Disease
Modalities
- X-Ray
- Computed Tomography (CT)
- Single-Photon Emission Computed Tomography (SPECT)
- Positron Emission Tomography (PET)
- Magnetic Resonance Imaging (MRI)
- Magnetic Resonance Spectroscopy (MRS)
Advanced Neuro-MRI Methods
- DTI – Diffusion Tensor Imaging
- NODDI – Neurite orientation dispersion and density imaging
- fMRI – functional MRI
- fcMRI – functional connectivity of resting state fMRI
- FDG-PET – Positron Emmision Tomography with 18F isotope
- vMRI – volumetric MRI
- WM/GM
- Cortical Thickness
- Structural Segmentation – Volume Measurement
- ASL – Arterial Spin Labeling for quantitative perfusion imaging
- SWI – Susceptibility weighted imaging