MASH & MASLD CRO Capabilities
As a therapeutically-aligned CRO, Medpace has the relevant and recent hepatology experience in indications such as metabolic dysfunction-associated steatohepatitis (MASH) and metabolic dysfunction-associated steatotic liver disease (MASLD) (formerly known as non-alcoholic steatohepatitis (NASH) and non-alcoholic fatty liver disease (NAFLD)) to successfully navigate the complexities and regulatory scrutiny often involved with these programs. Our experience, coupled with our strong relationships with KOLs and investigative sites and our in-house central lab and imaging core lab services, set you up for long term success.
MASH & MASLD Experts
The ability to recruit patients for hepatology studies in indications such as MASH and MASLD (formerly known as NASH and NAFLD) requires a comprehensive site feasibility assessment, a well-designed study, and established relationships with key opinion leaders and principal investigators. The in-house physicians, imaging specialists, and operational teams at Medpace can help sponsors successfully navigate the complexities and regulatory scrutiny involved with these programs. Our therapeutically-aligned teams bring a strong understanding of the key aspects of study design and patient eligibility criteria as well as the ability to manage potential challenges and logistical requirements associated with screening and enrolling these often difficult to recruit patient populations.
Deep Dive
Meet our MASH & Liver Disease Medical Experts
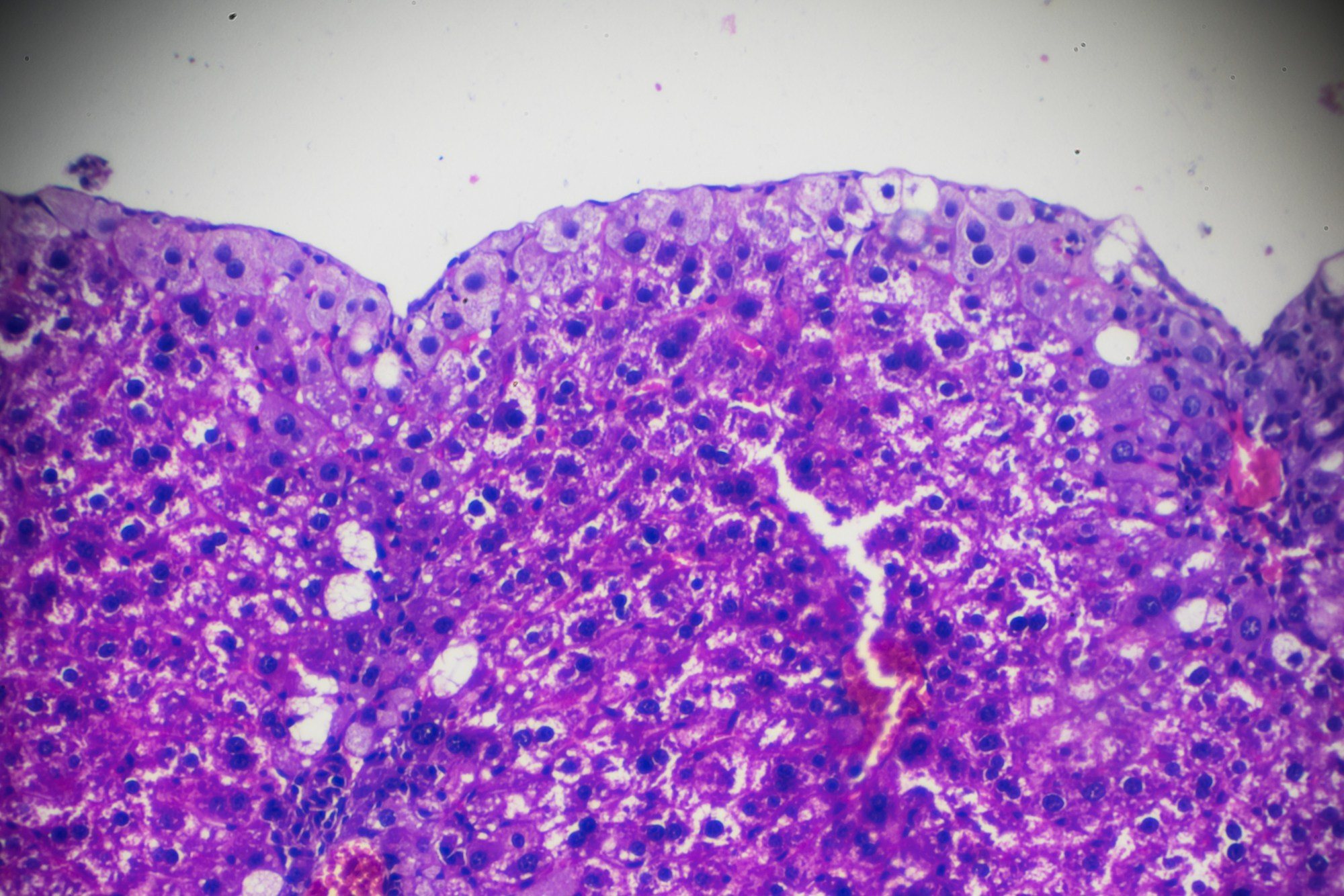
Integrated Central Laboratory
Through our wholly-owned laboratories in the US, Europe, China and Singapore, we have the global reach and capabilities to conduct MASH/MASLD studies in concert with the Medpace CRO or as standalone service. With over 30 years of extensive experience, we have validated a comprehensive menu of biomarkers associated with MASH: cytokeratin-18 fragments (M30 and M65), apolipoprotein A1, apolipoprotein B, leptin, adiponectin, resistin, free fatty acids, ghrelin, hsCRP, interleukin-6, and tumor necrosis factor-alpha. Medpace Labs’ test menu also includes validated assays used in MASH fibrosis scores such as Fibrotest/ FibroMax and ELF (Enhanced Liver Fibrosis).
Medpace histology offers routine and novel pathology services for your MASH studies, including tissue processing, sectioning, general and special stains, immunohistochemistry, insitu hybridization and digital pathology services with the ability to perform select assays in a CAP-accredited, CLIA-certified laboratory.
Specialized Imaging for MASH/MASLD Clinical Research
High-quality image acquisition and interpretation is crucial for the success of MASH/MASLD trials. Medpace Core Labs provides comprehensive central imaging services including site assessment, qualification and training, advanced data processing and blinded assessments. In particular, Medpace Core Labs has expertise with the implementation of various MR-Based acquisition techniques including Magnetic Resonance Spectroscopy (MRS), Proton Density Fat Fraction (MRI-PDFF; magnitude and complex) and Magnetic Resonance Elastography (MRE) to support imaging endpoints for MASH/MASLD trials using fully regulatory-compliant platforms.
Related MASH Insights
Webinar