Avoiding Radiation Toxicity – Understanding Dosimetric Endpoints and Challenges in Radiopharmaceutical Clinical Trials
As applications of radiation therapy continue to expand, biotechnology companies must improve find ways to improve their processes for applying it, safely, on a global scale, without excess toxicity to the patients.
In a newly released article, Medpace board certified radiation oncologists and Core Lab dosimetrists look into the future of radiopharmaceutical clinical development and provide radiopharmaceutical and radiation dosimetry concepts.

Radiation Therapy & Radiopharmaceuticals
An integrated Approach
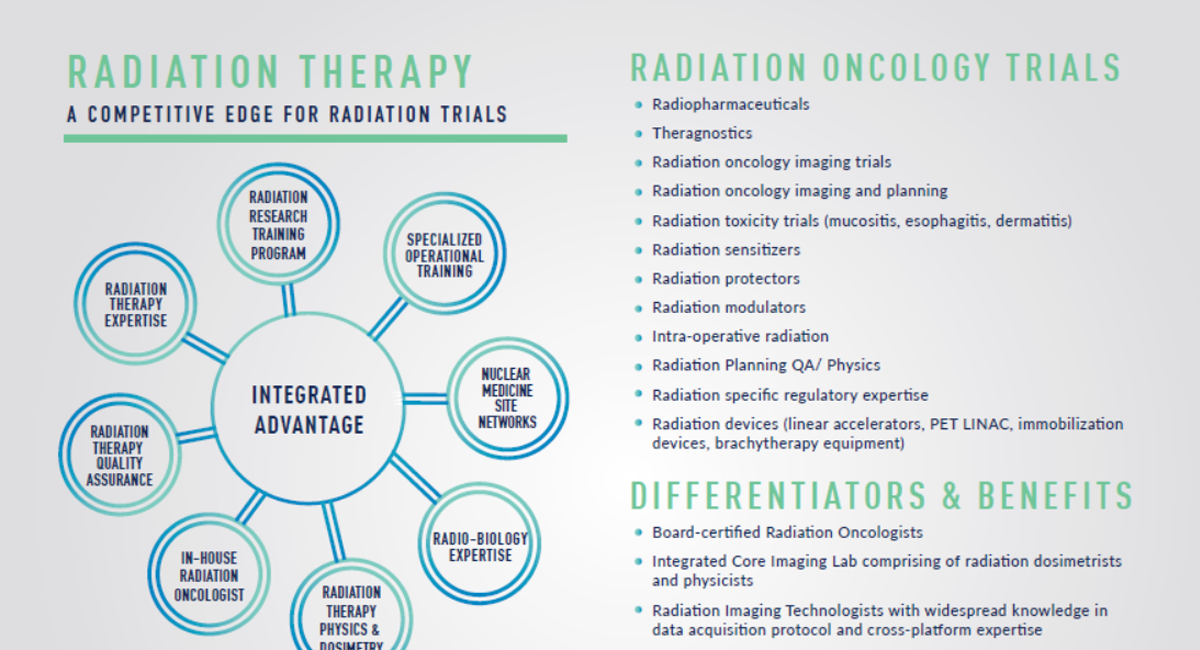
At Medpace, our integrated team of radiation oncologists and imaging experts accelerate the path to approval through the understanding of radiation biology, dosimetry, clinical, regulatory, operational, and imaging considerations that must be factored in when designing a radiation oncology trial. Our integrated advantages include:
- Cross-disciplinary radiation oncology capabilities providing state-of-the-art services for either stand-alone or fully integrated projects with Medpace CRO
- Board-certified radiation oncologists providing medical leadership to enhance and expedite radiopharmaceutical and radiation device development
- Comprehensive regulatory affairs and operational experts who work collaboratively with our medical team
- Fully-integrated Imaging Core Laboratory services, including expert guidance from our radiation physicists and dosimetrists