Organic Growth: Providing Stability and a Proven Operational Model that Preserves a Culture of Quality
While the CRO industry has grown primarily through mergers and acquisitions, Medpace has expanded through disciplined organic growth – expanding globally to operations in 36 countries and 2900 employees. Over the last two decades, Medpace has added specialized medical, regulatory, and operational experts, custom-built technologies and processes to best serve the needs of our Sponsors. The result is a culture built on quality that has not been disrupted by acquisitions, and delivers ongoing efficiencies and stability.
Our 27-year history with purposeful, organic growth provides consistency in leadership, deep institutional experience, and superior efficiencies as a top 10 CRO. Read on for details about our recent global expansions.
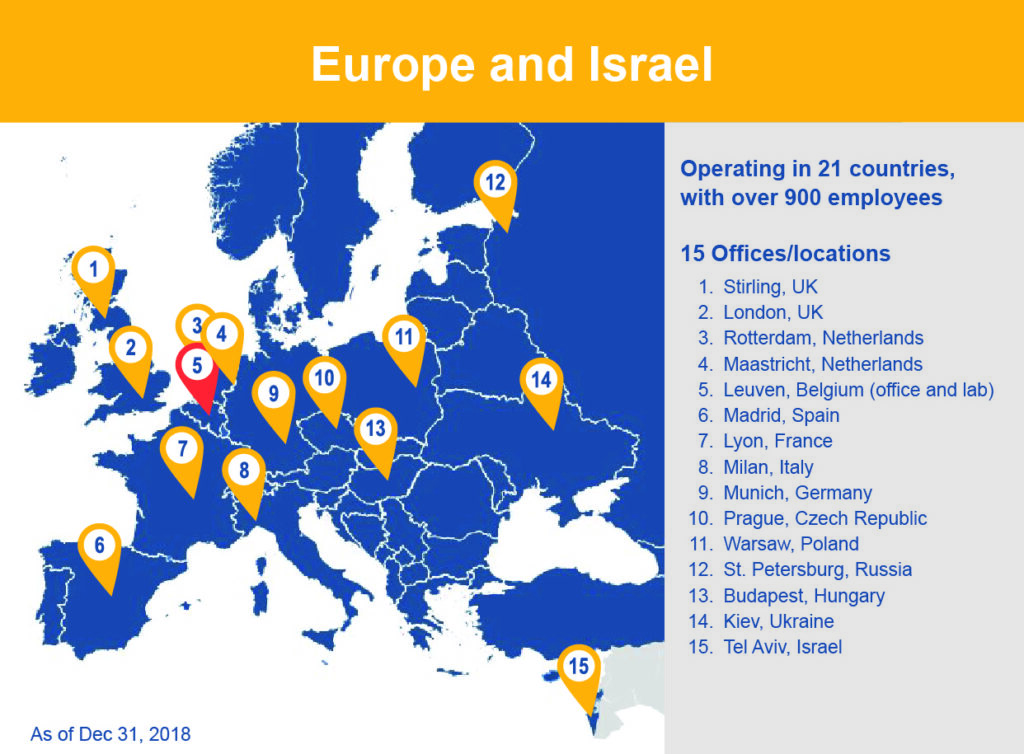
15 European Offices plus Central Laboratory and Biorepository Expansions
Medpace has nearly 15 years of experience working across the majority of European countries. We have over 900 employees across 21 countries with 15 brick and mortar office and lab locations. Our offices are strategically located to offer full-service clinical development services for a rapidly expanding global market. A significant number of clinical research professionals have been added globally with facilities expanding in Belgium, Czech Republic, France, Germany, Greece, Hungary, Italy, Netherlands, Poland, Spain, Ukraine, and the United Kingdom.
With substantial resources across Western, Central and Eastern Europe, Medpace is well-suited to seamlessly execute clinical research studies for biotech and biopharma in specialty fields such as advanced therapies and rare disease and in key therapeutic areas including hematology and oncology, cardiovascular, endocrine/metabolic, infectious diseases, neurology and psychiatry, nephrology, autoimmune, gastroenterology, and other growing therapeutic areas.
Our central laboratory in Belgium is also expanding. Molecular and genetics capabilities have been added, and a new logistics center will be operational by the summer of 2019 that doubles the square footage. Furthermore, the biorepository space is growing to 8,000 square feet and can provide storage capacity for more than four million samples. This biorepository facility supports specimen storage down to cryogenic temperatures and cryogenic shipping capabilities using cryoshippers. Sample management and storage areas are expandable to support the needs of our clients.
Acknowledging a new wave of technology currently being evaluated by biotechnology companies for innovative therapies, Daniel O’Leary, Sr. Vice-President, Medical Department for Medpace explained the goal of the expansions: “In response to the growing and dynamic market in Europe for innovative therapies such as CAR-T, immuno-oncology, and gene and cell therapies, Medpace continues to expand its in-house experts to support the needs around the globe. Our mission is to accelerate the approval of safe and effective medical therapeutics and our reputation for taking on some of the industry’s most complex and innovative clinical trials demands that we continue to expand our depth of medical expertise and provide both local and global support.”
Growing Offices across Europe and Israel
Expansion Spotlight – London

In 2018, Medpace moved its London office to centrally-located Vintners’ Place. The new office allowed for growth in Data Management, Bio-Statistics, Quality Assurance, Regulatory Affairs, Clinical Operations, and Safety. Together with the Stirling, Scotland office, Medpace provides local support for the thriving hub for R&D activity in the ‘Golden Triangle’ of London, Oxford, and Cambridge. Combined, both Medpace UK offices employ more than 200 employees and continues to grow.
Expansions Around the World
Europe is just one region of many growing to accommodate the global requirements of our clients. Medpace has also been expanding our footprint in Asia-Pac, Latin America and South Africa. You can stay up-to-date on our growth around the world and how we can support your global trials by visiting the Global Reach section of the web.