Experience, Global Reach, and Highly
Disciplined Execution
Medpace Clinical Trial Management leverages our unique partnering philosophy and team structure to deliver effective, end-to-end collaboration, training, and resource planning around the globe. Our dedicated teams are designed to serve as an extension of your team and provide unrivaled collaboration and support at every stage of the drug development process.
Sponsors Benefit From:
- A Proven Model: Our full-service operating model has been refined by over three decades of experience successfully executing the most scientifically and operationally challenging clinical trials without the disruption of mergers and acquisitions that are so common in the industry.
- Cross-Functional Expertise: Therapeutically focused and experienced clinical operations leadership, working closely with our medical, regulatory, and patient recruitment experts engage quickly and pool their collective knowledge to provide strategic guidance for your trial—driving quicker start-up times, superior quality, and the most efficient delivery at every phase of clinical development.
- Essential Partnerships: Comprehensive communication is utilized throughout all phases of the clinical trial to build and maintain successful partnerships—with both sponsors and sites.
- Dedication & Continuity: Shared common goals and expectations regarding drug development are the bedrock of our commitment to success. Continuity and peace of mind from development plan to product approval is made possible by dedicated, therapeutically focused leadership teams.
“Offering the full breadth of services in a centralized fashion – with one point of contact – was one of the things that was most attractive as we were evaluating different CROs.”
– VP, Clinical and Medical Affairs, Small Biotech
Global Leadership
Our Clinical Trial Managers (CTMs) ensure timely progress and overall quality of the deliverables for each trial. Working closely with our cross-functional project team, and other vendors, the CTM strategizes, plans, and coordinates all project activities to seamlessly execute the trial. Like the rest of our cross-functional team, our CTMs are therapeutically focused, managing trials across similar indications, allowing us to capitalize on knowledge and experience gained to drive efficiencies. Our clinical trial management team proactively monitors study milestones to ensure your trial is successful – delivered on time and on budget. For global studies with more than one geographic region involved, Medpace will deploy regional resources including CTMs to provide local expertise and support for trial execution.
Consistent Recognition for Outstanding Performance
Medpace has been the recipient of multiple industry awards based on feedback from sites and drug, biologic, and medical device sponsors, including recognition across all 5 categories in the 2024 CRO Leadership awards. These awards reflect our talented people, their dedication to our mission, and the depth of expertise that allows us to carry out complex and scientifically challenging programs.

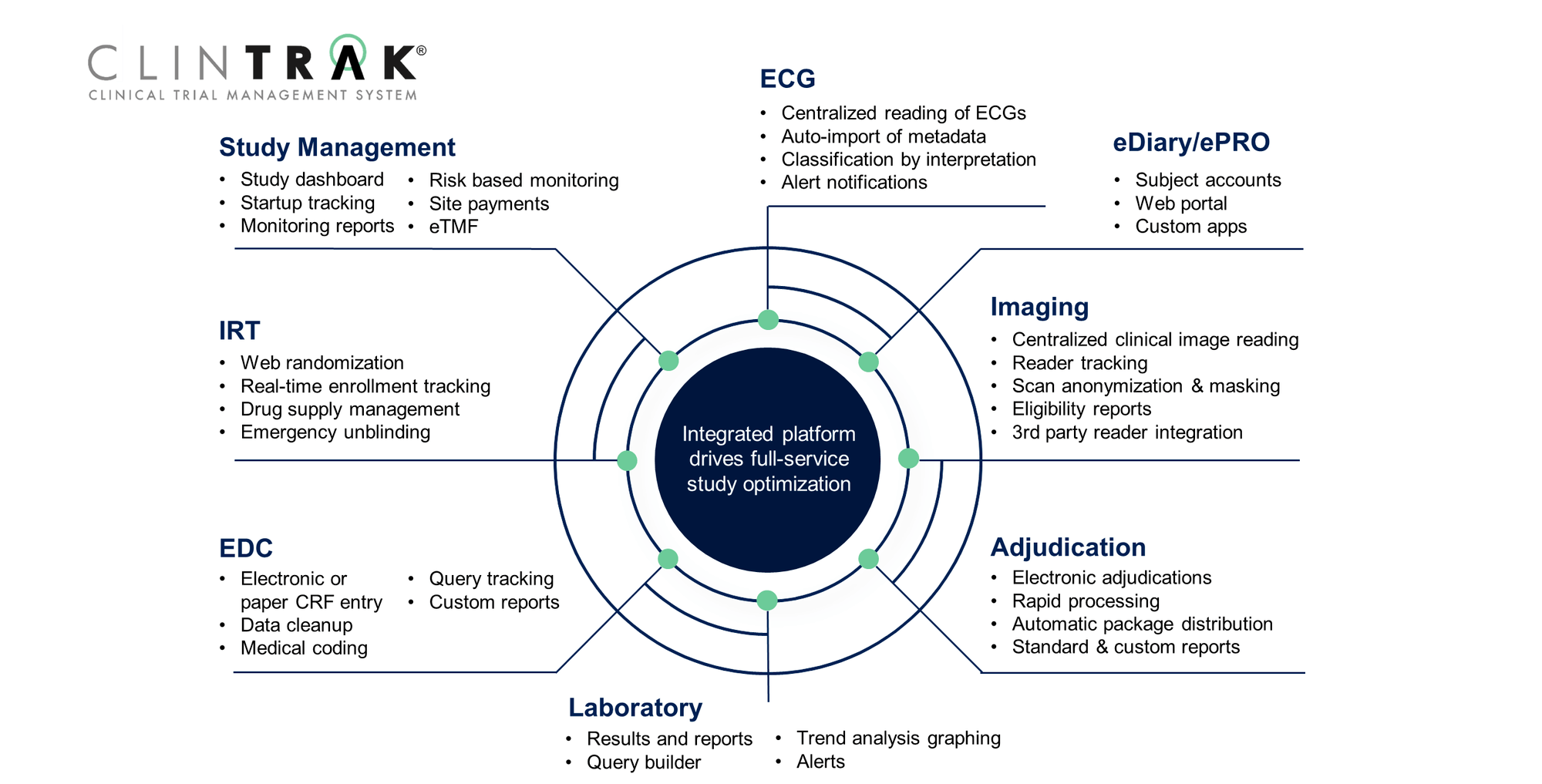
Fully-Integrated Technology
Every aspect of the clinical trial process is managed utilizing our sophisticated and fully customizable Clinical Trial Management System (CTMS), part of the ClinTrak® innovative suite of proprietary, leading-edge technologies. Developed and maintained by Medpace information technology experts, this study management system, which uses a common platform, provides:
- A decision support system and access to critical data allowing everyone involved- sponsors, investigators, and the Medpace team- to track, interpret, and communicate information in the most timely, secure, and cost-effective manner
- Sponsor oversight with access to real-time data and study metrics