Medpace Medical and Clinical Operations Experts Share Highlights from ESCMID Global 2025
In April 2025, Medpace infectious diseases and vaccines experts attended ESCMID Global in Vienna, Austria. As one of the most distinguished global events in microbiology and infectious diseases, this five-day conference brought together leading experts in the field. This year’s conference covered many current industry topics including the development of antibacterial and antifungal therapies, the global threat of antimicrobial resistance (AMR), the examination of precision medicine approaches, and advances in technology. Additionally, this year featured Pipeline Monday—a new track for academia, healthcare, industry, and policymakers—focused on the R&D pipeline.
Explore insights from Medpace medical and operational experts as they identify several of the key themes from ESCMID Global 2025.
Antibacterial Therapies
The conference highlighted key emerging trends and advancements in antibacterial therapies. Topics included the potential of emerging alternatives to traditional antibiotics, ongoing challenges in the evolving landscape of Gram-negative and Gram-positive bacteria, and new insights into interactions between pathogens and their host—offering new opportunities to treat bacterial diseases.
Antifungal Drugs
A growing concern in the industry is the rise of fungal infections, which remain difficult to combat and treat. Progress in antifungal drug development has lagged in the past due to the technical and clinical challenges involved in identifying fungal infections. However, two new broad spectrum antifungal drugs have been approved recently and look promising. Meanwhile, antifungal resistance is a growing issue that underscores the need for a better understanding of their prevalence and implications for effective treatments.
Challenges of Finding New Anti-Infectives Against Bacterial Pathogens
AMR is an urgent and growing public health concern that requires innovative approaches to combat, including the development of new treatment options and improved diagnostics. According to the CDC, there are more than 2.8 million AMR infections in the United States each year. AMR is a global threat and regions such as Asia face heightened risk due to high population density, fewer antibiotic regulations, poor sanitation, and limited access to healthcare. At the conference, discussions focused on strategies to preserve antibiotic effectiveness, the potential role of vaccines in reducing AMR and the spread of drug-resistant pathogens, as well as the broader implications of AMR for the future of modern medicine.
It was expressed as a recommendation from ECIL (European Conference on Infections in Leukemia) that new antibacterial drugs can be used for the empirical or targeted treatment in immunocompromised patients (e.g., febrile neutropenia), even if the specific drug has not been approved for that specific indication, considering the antibacterial has shown good efficacy and safety in other indications.
Viral Prevention/Prophylaxis
Preventative strategies for viral diseases were a key focus at the conference, emphasizing the importance of vaccines in minimizing outbreaks and reducing disease burden. Sessions highlighted cutting-edge innovations in preventative measures against RSV, influenza, HIV, and COVID-19. Industry leaders also explored challenges related to varying immune responses to vaccination, including immunosenescence—the age-related decline in immune function.
Next-generation sequencing was another focus at the conference, with sessions directly targeting metagenomics for infectious disease diagnostics. This technology holds crucial application value in infection pathogen diagnosis, providing a scientific basis for clinicians to develop targeted antimicrobial strategies, thereby enhancing treatment effectiveness and improving patient outcomes.
“At the ESCMID Global conference, it was exciting to see continued innovation in scientific advancements driving progress in the prevention and treatment of infectious diseases. As a full-service CRO, Medpace is committed to accelerating the development of safe and effective medical therapeutics to prevent and treat infections, ultimately improving the lives of patients worldwide.”
– Carrie Sheil, Executive Director, Clinical Trial Management, Medpace
As we reflect on the latest trends in ID&V clinical development, we also look forward, preparing for the next wave of innovations in the industry. New innovations, advancements in technology, and the growing ID&V clinical development market offer the community renewed hope for advancing global health.
The complex and dynamic nature of infectious diseases demands an experienced CRO partner with deep medical expertise across various indications and an understanding of the constantly evolving landscape to effectively conduct global ID&V clinical trials. Our thorough medical, operational, and regulatory understanding of the complexities of infectious diseases trials allows us to identify potential pitfalls early and implement strategies to avoid them, helping to minimize risk and accelerate bringing new therapies to market.
Interested in learning more? We welcome the opportunity to discuss your upcoming clinical development plans. Contact our ID&V experts today.
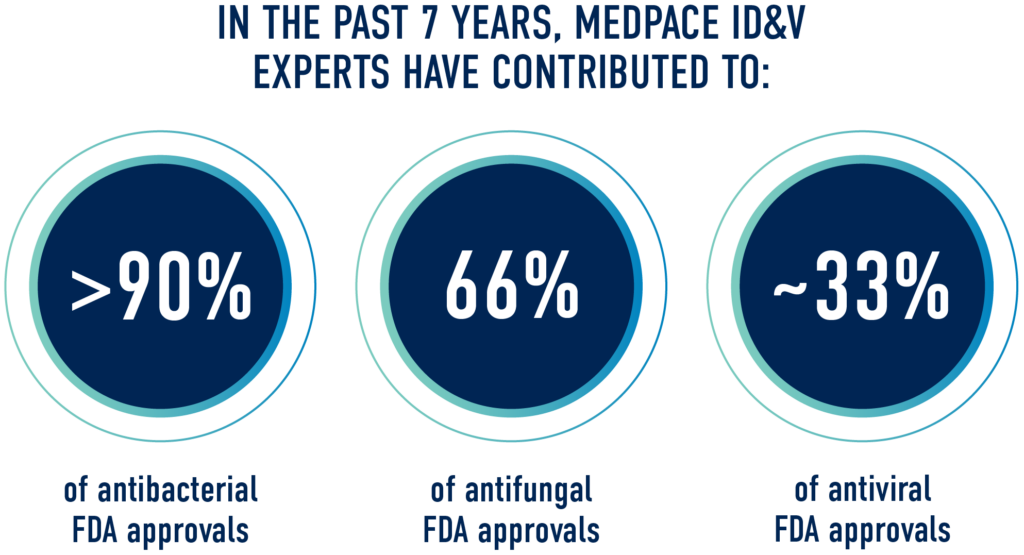
An Industry Leader in ID&V Clinical Development
Medpace’s specialized cross-functional team of experts have extensive experience executing global trials in antivirals, antibacterials, antifungals, vaccines, and immunotherapies spanning adult and pediatric patients, as well as special at-risk populations. Our experienced medical leadership team, integrated laboratories and clinical trial management system, and extensive site and investigator relationships produce a truly seamless drug development process.