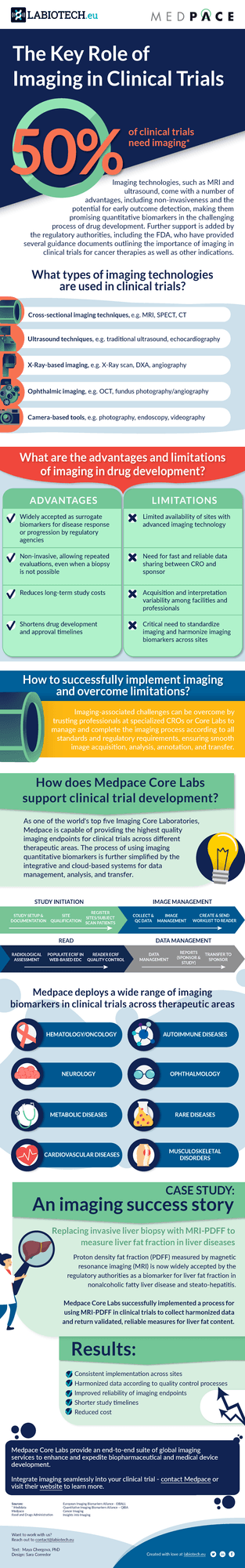
How to Successfully Implement Imaging in Clinical Trials
Imaging technologies such as MRI, PET, and DXA, can be used as quantitative biomarkers in clinical trials. Utilizing these non-invasive techniques can reduce the overall cost of a study and shorten drug development times. While efficiencies in the trial are certainly an advantage, using imaging as a biomarker is not without limitations. To manage these hurdles, many biotech companies partner with a CRO with an integrated Core Lab to manage, analyze, and transfer the data.
In one example using MRI-PDFF, Medpace demonstrated quantitatively that at least 3 regions of interest (ROIs), produces a reliable and accurate alternative to 9 ROIs. This allows for consistent implementation across sites, improved reliability of endpoints, and reduction in overall study cost and timelines.
In this new infographic, we explore the different types of imaging techniques, their advantages, limitations, and how they can be successfully implemented in clinical trials.
Learn more about the Medpace Core Labs or contact us about an upcoming trial.