A major takeaway from ILC 2020 were the novel findings regarding PDFF as a key MRI biomarker in NAFLD and NASH studies. As demonstrated in multiple clinical trials1,2 by Dr. Rohit Loomba from University of California, San Diego, most of the subjects under active treatment were classified as MRI-PDFF responders (relative decline in liver fat ≥ 30%) compared to placebo after few weeks only.
It has been reported3,4 that an early MRI-PDFF response of ≥ 30% fat reduction was also associated with later improvement in biopsy components, and MRI-PDFF improvement was associated with NASH resolution relative to placebo. These key findings demonstrate the benefit of imaging in clinical trials and especially the high potential of the MRI-PDFF assessment as a key endpoint for studies.
Three is Enough
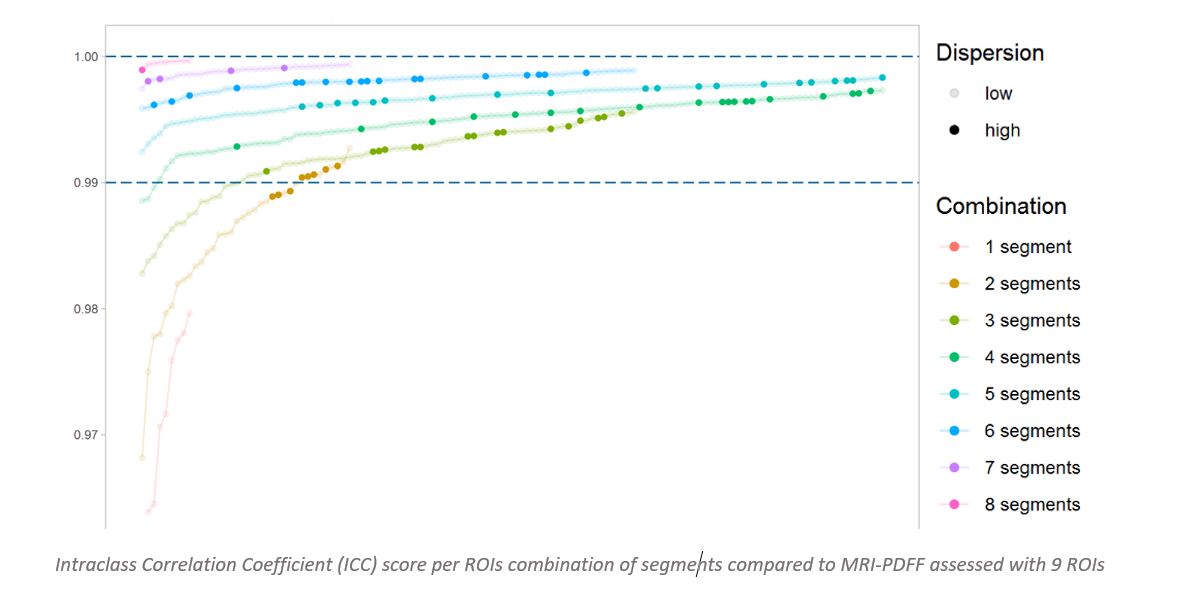
This year, Medpace Imaging Core Labs had the opportunity to bring our expertise to the forefront with a novel poster presentation: “Three is enough: Optimization of regions of interest selection for accurate estimation of proton density fat fraction (PDFF) in liver from magnetic resonance images (MRI)”. We demonstrated quantitatively that at least 3 regions of interest (ROI), with the greatest dispersion over the Couinaud segments, produces a reliable and accurate alternative to 9 ROIs. View the poster here.
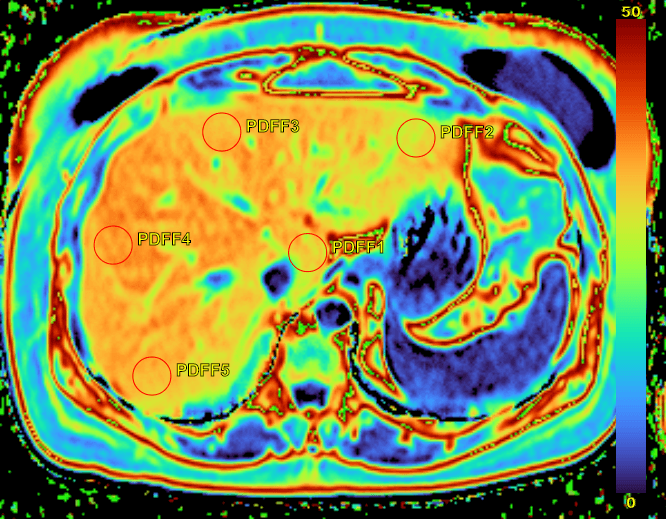
For NAFLD and NASH clinical trials, one of the key imaging biomarker is the estimation of MRI-PDFF. The measurement of this endpoint is critical to accurately assess the evolution of MRI-PDFF in liver throughout the course of the study. One method proposed to assess MRI-PDFF from an MRI scan is to draw 9 ROI on the generated MRI PDFF map in each of the Couinaud segments (functional liver anatomy)5. This is demonstrated in the image below with ROI highlighted in red. Then, the mean of the pixel intensities from each ROI is used to estimate MRI-PDFF for the MRI scan. Unfortunately, this procedure may be biased by some MR image artifacts or complicated by the identification of the Couinaud segments in the context of multi-centered clinical trial.
Medpace Imaging Core Lab demonstrated quantitatively that at least 3 ROIs, selected with care to avoid large blood vessels or ducts and to achieve the greatest dispersion over the Couinaud segments, produces a reliable and accurate alternative to 9 ROIs.
Learn more about Medpace’s imaging capabilities.
- VK2809, a novel liver-directed thyroid receptor agonist, produces durable reductions in liver fat in patients with non-alcoholic fatty liver disease: Results of 4-week follow-up assessment from a 12-week Phase 2 randomized, placebo-controlled trial
- Novel first-in-class, fatty acid synthase inhibitor, TVB-2640 versus placebo demonstrates clinically significant reduction in liver fat by MRI-PDFF in NASH: A phase 2 randomized controlled trial (FASCINATE-1)
- Magnetic resonance imaging-proton density fat fraction (MRI-PDFF) to predict treatment response on NASH liver biopsy: a secondary analysis of the resmetirom randomized placebo controlled Phase 2 clinical trial
- Multifactorial Effects of AXA1125 and AXA1957 Observed on Markers of Metabolism, Inflammation and Fibrosis: A 16-Week Randomized Placebo-Controlled Study in Subjects With Nonalcoholic Fatty Liver Disease (NAFLD) With and Without Type 2 Diabetes (T2D)
- Hong CW, et al. Optimization of region-of-interest sampling strategies for hepatic MRI proton density fat fraction quantification. J Magn Reson Imaging. 2018;47:988-94.