Global MASH Clinical Trial Imaging Operations
- Offering advanced quantitative imaging biomarkers for metabolic dysfunction-associated steatohepatitis (MASH) (formerly known as non-alcoholic steatohepatitis (NASH))
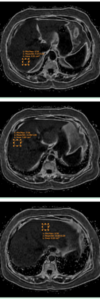
- Volumetric measurements for liver and spleen
- Fat content using MRI and/or MRS proton density fat fraction estimates (MRI-PDFF)
- Fibrosis/stiffness using MR elastography and/or FibroScan® acoustic elastography
- Full-service multi-platform imaging core lab (GCP & CFR-Part 11)
- Global site management
Deep & Broad MASH Imaging Experience
- Global reach with trials at more than 200 sites in more than 1000 patients
- Multi-site management and QA for trials with MRE, MRS, MRI and FibroScan® biomarkers for NASH
Maximize Consistency of Image Acquisition within
and across Sites
- Define and verify scanners, acquisition parameters, anatomical coverage, and scanning protocols
- Establish procedures for site qualification, routine quality assurance, and quality control
- Audit trail from enrollment to image upload and verification
Ensure Consistency of Image Review and Analysis
- Experienced project managers and technologists verify image uploads and quality
- Qualified, board certified centralized image readers/reviewers
ClinTrak Imaging® – Medpace Clinical Trial Management System
A powerful, proprietary image management software program purpose-built for supporting clinical trials
- Full integration of CTMS with imaging workflow (industry leader)
- Team of dedicated in-house developers
- Secure electronic transfer images/files
- 21 CFR Part 11 compliant
- 24/7 site support in various languages
- Image/scan tracking and archiving
- De-identification/masking
- Customized imaging eCRF
- Data management/query resolution and tracking
- Integrated image virtualized workstation
- Real-time web-based status reporting