ClinTrak® – a Study Management System Using a Common Data Platform
Clinical development projects require a common data platform to facilitate team coordination and provide decision support for sponsors and sites – ensuring global teams are focused and organized for maximum efficiencies. Making the wrong decision based on poor quality data is a main reason studies fail.
Choosing the right development partner with an integrated, robust technology platform is a critical step for ensuring successful studies are conducted at the highest level of data integrity.
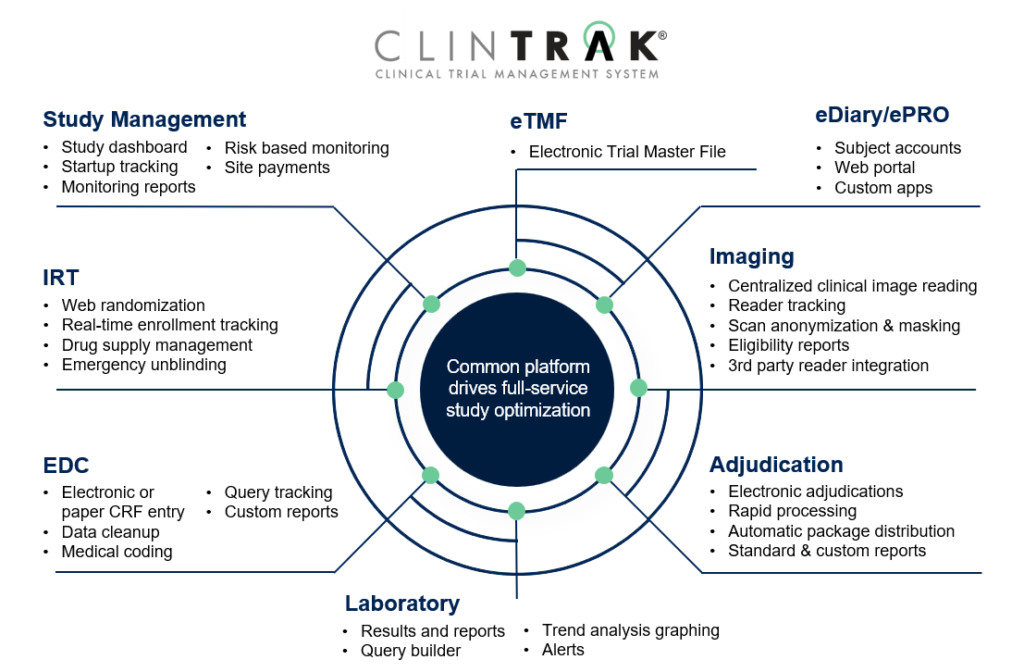
ClinTrak® Modules
Clinical Trial Management System (CTMS)
This study-specific web-portal provides the team with a set of collaboration pages for secure posting and sharing of study documents.
Interactive Response Technology (IRT)
Provides global access to web-based interfaces for authorized personnel, allowing real-time subject tracking, inventory management, and randomization.
Electronic Data Capture (EDC)
Provides a centralized location for the study team to review real-time case report form (CRF) data.
Laboratory Information Management (LIMS)
Full scale decision support management system that gives you the power to compare customized results from patients across the globe.
Imaging Management
Integrates image tracking, quantitative and qualitative analysis, and data management to store and manage data from all reading centers.
ePRO/eCOA/eDiary
Allows for the safe and secure collection of PRO and eCOA data directly from patients through multiple platforms.
Wearable Biosensor Technology
Wearables and other patient-centric portable devices that remotely collect individual biometric data are transforming clinical trials. The integration of digital biometrics from wearables and remote devices into clinical study data delivers several advantages to patients, sites, and Sponsors.

Apps Supporting Patients and Sites
In today’s digital world, mobile apps have become an essential part of daily life, and clinical research is no different. Seamlessly integrating into the digital landscape, our suite of custom-built apps offers convenience, cleaner data, and enhanced user experiences. Through these dedicated tools, we can help improve both patient and site engagement – leading to higher recruitment and retention rates.