The Future of Wearables in Clinical Trials
Wearables and other patient-centric portable devices that remotely collect individual biometric data are transforming clinical trials. These devices can be used to evaluate the effectiveness of a medical therapy while delivering many other vital operational and analytical advantages.
Traditional approaches have relied primarily on intermittent assessments of biometric data, usually performed at the trial site. With the development of continuous remote biosensing technologies, wearable devices can support virtual, hybrid, and decentralized trials.
Incorporating New Data Streams While Reducing Costs
Integration of digital biometrics from wearables and remote devices into clinical study data delivers several advantages to patients, sites, and Sponsors. Continuous collection of biosensor data can support digital endpoints and a more compelling regulatory case. This approach is supported for remote ECG recording by professional rhythm organizations such as the Heart Rhythm Society and European Heart Rhythm Association.
Data streams from digital biosensors can impact study design, patient selection, and go/no-go decisions.
- Decreased site visits: reduced site and resource burden leads to cost savings
- Improvement of patient recruitment and compliance: fewer office visits and improved adherence with trial protocol requirements leads to reduced protocol deviations
- Better monitoring of patient’s overall health for site coordinators and investigators
The Medpace Advantage to Connected Devices
Wearable biosensor technologies selected and vetted by Medpace’s therapeutically-aligned teams meet tailored study design requirements, streamline vendor management, and accelerate study start-up. Medpace seamlessly collects, harmonizes, and integrates data from wearables and remote devices into your clinical study as part of our full-service offering.
Benefits include:
- One-stop shopping with assurance of device selection, logistics and availability to sites
- Faster and more efficient execution leveraging relationships and experience with device manufacturers
- Technology and expertise to support integration, centralized review and analysis, harmonization, and visualization of study data and management
- Secure data capture and transfer using our vendor-neutral platform to bridge the communication from device to database
- Expertise to monitor, analyze, and transform data from essential biomarkers to support regulatory review
Medpace Core Lab Integration
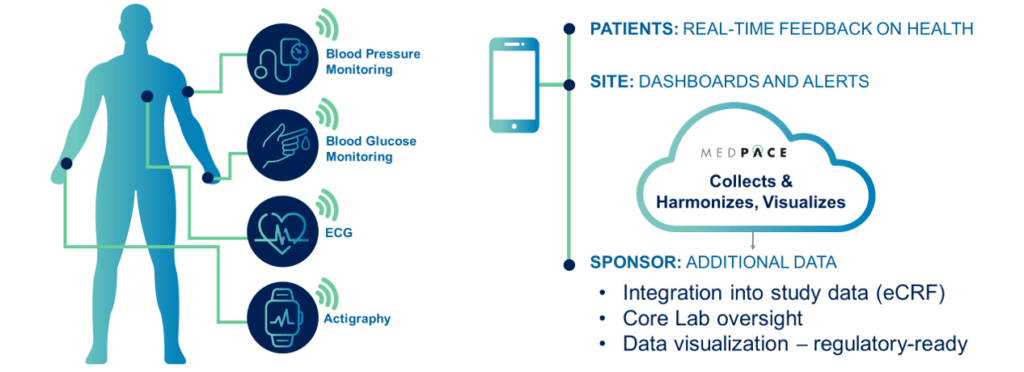
Medpace Core Labs provide another level of oversight and analysis for essential biometric data, such as ECGs, blood glucose, cardiac output, actigraphy, volume status, and vital signs.
Our integrated core labs provide an end-to-end suite of global imaging and cardiac safety services to enhance and expedite biopharmaceutical and medical device development. Biosensors produce prodigious amounts of previously untapped health data for clinical studies, and Medpace can integrate this data into the overall study and harmonize it to present strong regulatory cases.
Data Protection
Data from patient biosensors requires up-to-date security infrastructure and controls, data transfer protection, cybersecurity software, firewalls, and data Breach and Attack Simulations (BAS). MCL provides secure systems to manage, report and visualize remote biosensor data in a format that expedites regulatory review.
Data breach detection and correction ensure data security and regulatory compliance. BAS test security controls of the Medpace environment to guard against the latest threats to cyber and data security.